Introduction
T-cell prolymphocytic leukemia (T-PLL) is a rare and aggressive malignancy with limited treatment options, frequent relapse, and poor long-term survival. Allogeneic hematopoietic cell transplantation (allo-HCT) is reported to be effective in T-PLL yielding durable remissions and improved survival. Rates of survival and non-relapse mortality vary; data is scant and limited to small retrospective series. Whether regimen intensity - a modifiable factor- is associated with outcomes is unknown. Here, we report an analysis of patients with T-PLL undergoing allo-HCT in 87 centers using data reported to the Center for International Blood and Marrow Transplant Research (CIBMTR).
Methods
A retrospective multicenter study was conducted utilizing the CIBMTR dataset, which captures all consecutive patients transplanted in participating centers. The study population included patients aged 18 or older with T-PLL who underwent allo-HCT between 2008 and 2018. All donor types, graft sources, and conditioning intensities are summarized (Table 1). Cox regression analysis was used to assess overall mortality, treatment-related mortality (TRM), relapse or progression, disease-free survival (DFS) and acute and chronic graft-versus-host disease (GVHD). Due to the strong correlation between regimen intensity and patients' age, a combined variable was constructed, and in all models, interactions between age/regimen and significant covariates were examined.
Results
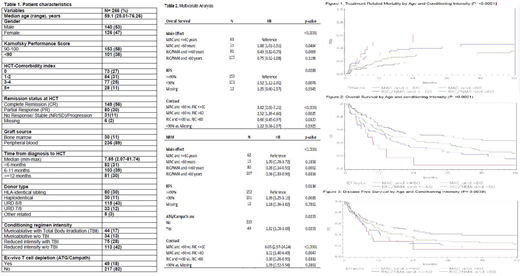
A total of 266 pts were identified and included in this analysis (Table 1). Median follow up of survivors was 49 months (range 3-117); the completeness of survival data out to four years was 95%. In univariate analysis, the 4-year overall survival (OS) rate was 30% (95% confidence interval [CI]: 23.8-36.5%). The 4-year DFS, relapse, TRM rates were 25.72% (95%CI: 20-32%), 41.9% (95%CI: 35.5-48.4%), and 32.4% (95%CI: 26.4-38.6%), respectively. Cumulative incidences of grade 2-4 acute GVHD at day +180 and chronic GVHD at 1-year were 22.5%% (95%CI: 16.8- 28.9%) and 38.3% (95%CI: 32.9-44.9%), respectively.
Multivariate analyses was performed using age (Age >60 vs Age ≤60) and conditioning intensity (myeloablative [MAC] vs. reduced intensity/non-myeloablative [RIC/NMA]) as main effect (Table 2). Poor performance status was associated with inferior TRM, DFS and OS. Compared to MAC/Age ≤ 60, RIC/NMA/Age≤ 60 correlated significantly with less TRM (hazard ratio [HR] 0.28, 95% CI 0.14-0.55, p= 0.0022, superior DFS [HR 0.53, 95% CI 0.35-0.81, p= 0.0029; (Figure 3) and OS HR 0.49, 95% CI 0.32-0.75, p= 0.0009; (Figure 2). Similar trend was observed for RIC/NMA/ Age> 60 for all 3 outcomes but did not reach statistical significance.
Remission status at time of allo-HCT (stable disease or progression vs. complete remission) correlated with relapse (hazard ratio [HR] of relapse=2.13, 95%CI 1.23-3.71, p=0.0072), however degree of response (partial response vs. complete remission did not significantly correlate with relapse (HR of relapse=1.41, 95%CI 0.91-2.17, p=0.1257). In vivo T cell depletion with ATG (N=47) or alemtuzumab (N=2) was associated with worse TRM [HR 1.82, 95% CI 1.08-3.08, p= 0.0255] and inferior DFS [HR of death or progression=HR 1.50, 95% CI 1.05-2.15, p= 0.0276]. The most common cause of death was disease relapse (52%) followed by infection (15%) and GVHD (13%).
Conclusion
This CIBMTR study, with the largest cohort of allo-HCT in T-PLL to date, demonstrates allo-HCT is effective in providing durable remissions. MAC and poor performance status were predictive of inferior TRM and OS. Disease relapse was most common cause of death, and pre-allo-HCT remission inversely correlated with relapse rate. The data suggest that reduced intensity conditioning and avoidance of post-transplant ATG/alemtuzumab may be beneficial. Novel strategies are needed both pre and post-allo-HCT to further improve outcomes.
Dholaria:J&J: Research Funding; bms: Research Funding; Takeda: Research Funding; Poseida: Research Funding; Angiocrine: Research Funding. Jagadeesh:Verastem: Membership on an entity's Board of Directors or advisory committees; Debiopharm Group: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; MEI Pharma: Research Funding; Regeneron: Research Funding. Oran:Celgene: Consultancy; ASTEX: Research Funding; Arog Pharmaceuticals: Research Funding. Nakamura:Viracor: Consultancy; Celgene: Other: Support on seminar; Magenta Therapeutics: Other: Advisory board meeting; NapaJen Pharma: Consultancy; Alexion: Other: Support on a meeting presentation; Merck: Other: advisory board meeting; Kadmon Corporation: Other: Advisory board meeting; Kyowa-Kirin: Other: Support on a meeting presentation. Sauter:Spectrum Pharamaceuticals: Consultancy; Sanofi-Genzyme: Consultancy, Research Funding; Gamida Cell: Consultancy; Novartis: Consultancy; Genmab: Consultancy; Precision Biosciences: Consultancy, Research Funding; Kite - a Gilead Company: Consultancy; Celgene: Consultancy, Research Funding; GSK: Consultancy; Bristol-Myers Squibb: Research Funding; Juno Therapeutics: Consultancy, Research Funding. kharfan-Dabaja:Daiichi Sankyo: Consultancy; Pharmacyclics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal